

In the above reaction, the total 𝐴 on both sides of the equation is 14 and the total 𝑍 T o t a l t o t a l t o t a l t o t a l r e a c t a n t s p r o d u c t s =. In this reaction, carbon-14 transforms intoįor a nuclear reaction to be balanced, the total 𝐴 and the total 𝑍 must be the same
#Which change occurs during a nuclear fission reaction plus#
(sum of neutrons plus protons), and 𝑍 is the charge of the particle (number of protons for nuclei).įor example, the reaction that is used in carbon dating is shown below. Symbol for the particle (such as the atomic symbol), 𝐴 is the mass number

We will use nuclide notation X, where X is the To represent particles participating in the reaction, Nuclear reactions can be represented in a reaction equation style. They give different products with different isotopes of the same element. They give the same products of the reaction even with different isotopes They can cause a transformation in an element’s isotope or can causeĪn element to transform into another element entirely. They do not cause an element to transform into another. They take place between the nuclei of atoms.
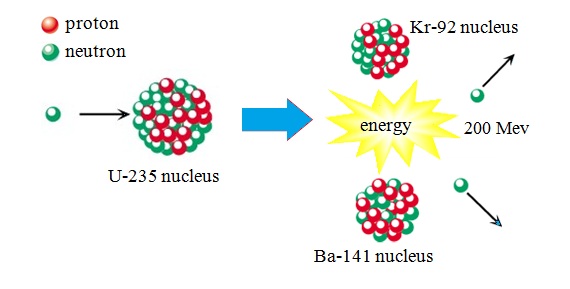
They take place between the electrons of the atom’s outermost shells. These differences between chemical reactions and nuclear reactions can be Will not making it useless for determining the age of anything that is discovered by archeologists. For example, carbon-14 is used forĭetermining the age of carbon-based archeological samples because it slowly transforms into nitrogen-14, but carbon-12 Isotope, and this will make it more or less likely to undergo a nuclear reaction. One isotope can contain more nucleons than another Nuclear reactions because they have different numbers of neutrons. Isotopes have similar chemical reactions because they have the same number of electrons, but they will have different The amount of energy released during conventional chemical reactions. The transformation processes are usually accompanied by extremely large changes in energy that can be up to a million times greater than Often result in an atom of one element transforming into an atom of a completely different element. During a nuclear reaction, neutrons and protons can change and entire nuclei can combine or break apart.


 0 kommentar(er)
0 kommentar(er)
